A Case of a 16-Year-Old Patient With Chronic Invasive Aspergillosis in the Trachea Treated With Segmental Tracheal Resection and Cricotracheal Anastomosis
Article information
Abstract
Chronic invasive aspergillosis is a life-threatening disease, especially in immunocompromised patients. The diagnosis and treatment of tracheal aspergillosis (TA) are challenging because of its rarity and nonspecific clinical presentations. The treatment standard of TA has been medical treatment like other forms of invasive aspergillosis, but patients with medically resistant TA require surgical intervention. We demonstrated a successful surgical outcome of chronic invasive TA in a 16-year-old patient with immunocompromised status related to acute myelocytic leukemia.
INTRODUCTION
The chronic invasive aspergillosis of the upper airways has been associated with patients with the immunocompromised state, including human immunodeficiency virus infection, hematologic malignancy, or long-term use of immunosuppressive agents [1].
Tracheal aspergillosis (TA) is regarded as an unusual form of invasive aspergillosis [2], and the main predisposing factors of patients with TA are similar to other forms of invasive aspergillosis, including hematologic malignancies, neutropenia, solid organ or hematopoietic stem cell transplantation, and chronic corticosteroid therapy [3]. Previous reviews of TA reported a high mortality rate of 40%–70%, and neutropenia and acute respiratory failure as an initial presentation of the disease constitute the main predictors of mortality [2,4].
The clinical presentations of TA have been reported to be variable and nonspecific, which results in delayed diagnoses. TA’s common symptoms included fever, cough dyspnea, and hemoptysis reported in 11.5% to 26.3% [2,5,6]. In addition, TA may present as a whitish plaque with pseudomembrane, diffuse or focal ulceration in the bronchoscopic examination [2,7,8].
There is no specific recommendation for the management of TA. Voriconazole alone or in combination with other medications has been used in clinical practice [2], but most patients improved when treated appropriately with antifungal agents in combination with surgical removal of infected tissue [5].
We presented a case of a patient with chronic invasive TA resistant to medical treatment, who revealed nonspecific respiratory symptoms, including hemoptysis. In this report, we demonstrated the successful outcome of surgical intervention for this patient and presented a review of the literatures.
CASE REPORT
A 16-year-old male was diagnosed with relapsed acute myeloid leukemia, and he had been treated with chemotherapy (idarubicin, fludarabine, cytarabine) with plans of bone marrow transplantation, serum galactomannan level checked during the chemotherapy was reported to be negative.
The patient presented with globus sensation and hemoptysis two months after the chemotherapy. He had a fever of 37.8°C, and the laboratory results included a leukocyte count of 2430 cells per cubic millimeter (absolute neutrophil count of 1750 cells per cubic millimeter), and serum galactomannan level was increased to 0.66. A plain chest radiograph revealed no demonstratable abnormality.
Positron emission tomography performed for preprocedural evaluation of bone marrow transplantation presented with a focal hypermetabolic lesion (SUVmax=7.7) of the trachea at the thyroid level (Fig. 1A). Neck ultrasonography demonstrated a 1cm endotracheal lesion at the anterolateral tracheal wall (Fig. 1B), and bronchoscopic examination showed focal whitish plaque attached to the trachea (Fig. 1C). Biopsy reported the presence of fungal hyphae, morphologically aspergillus species.

Image and endoscopic findings of TA. A: Positron emission tomography showed a focal hypermetabolic lesion in the cricoid and trachea. B: Ultrasonography demonstrated a 1 cm endotracheal lesion at the left anterolateral tracheal wall of thyroidal level. C: Bronchoscopic examination revealed a nodular yellowish mucous plug which formed pseudomembrane. D: Postoperative endoscopic examination showed mucosal healing status without infection sign. TA, tracheal aspergillosis.
Voriconazole was administered at a target dose of 7.8 mg/kg/dose initially but was lowered to 6 mg/kg/dose over 3 hrs due to blurred vision and glares. Ocular symptoms were resolved, and the maintenance dose was targeted at 4 mg/kg/dose the next day. Because medical treatment could not be maintained due to side effects, tracheal resection with cricotracheal anastomosis was performed. The perichondrium of anterior trachea cartilage was found to have degenerated, and the first to second tracheal rings containing the whitish plaque were resected with adjacent thyroid tissue (Fig. 2A). Then, end-to-end anastomosis of tracheal resection margin was made, and tracheostomy was formed. The final pathology revealed fungal hyphae in periodic acid-Schiff stain and Grocott’s methenamine silver stain in favor of invasive aspergillosis (Fig. 2B and C).
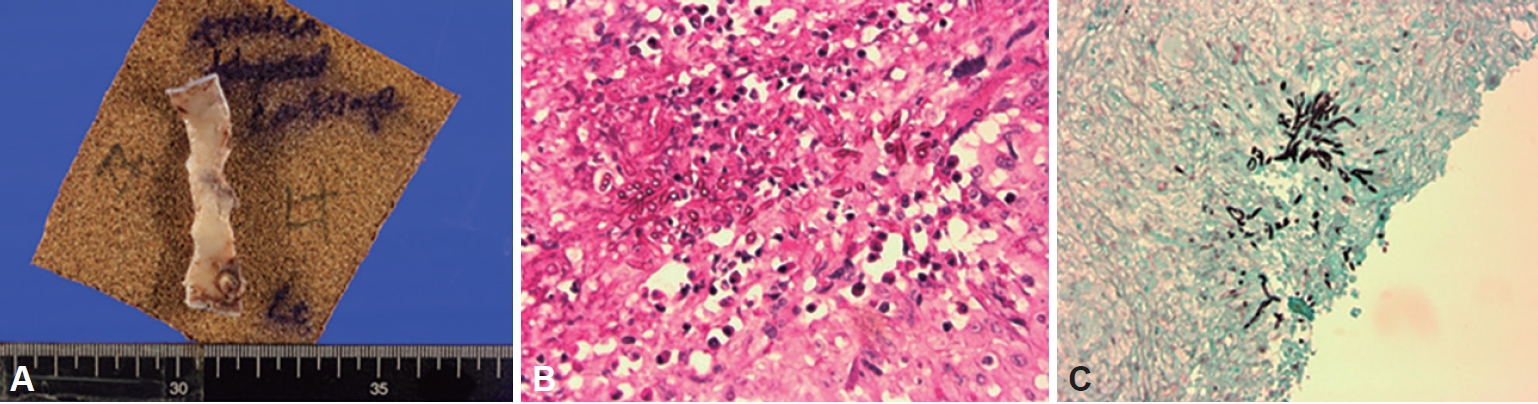
Pathologic findings of TA. A: Gross appearance of the resected trachea. B: A microscopic section is demonstrating branching Aspergillus hyphae invading tracheal mucosa (Periodic acid-Schiff stain, magnified ×200). C: Fungal hyphae invasion to tracheal mucosa (Grocott’s methenamine silver stain, ×100). TA, tracheal aspergillosis.
Bronchoscopic examination 3 days after the surgery showed no remanent lesion in the trachea (Fig. 1D). There was no other perioperative complication, and he was discharged ten days after the operation. Posttreatment serum galactomannan level decreased from 0.66 (before surgery) to 0.17 (1 week after surgery) and finally to 0.15 (3 weeks after surgery). He successfully underwent allogeneic hematopoietic stem cell transplantation 12 days after the operation, and Voriconazole 200 mg twice a day was maintained for three months postoperatively. The patient is still being followed and remains free of any recurrence of invasive fungal infection.
DISCUSSION
Invasive aspergillosis is histologically characterized by invasion of airway mucosa by fungal hyphae that can result in tissue ulceration or the formation of pseudomembranes consisting of necrotic epithelium overlying injured mucosa [5]. Invasive aspergillosis could occur anywhere in the airway, and diagnosis is challenging when the disease is located at the trachea and bronchus because of its rarity and nonspecific symptoms [5]. TA is an unusual form of invasive aspergillosis that occur in the airway being reported in less than 10% of cases, and patients with malignancies and solid organ transplant recipients were shown to be more susceptible to TA [2].
Previous studies reported that patients with neutropenia and initial presentations as acute respiratory failure might have a higher mortality rate. The formation of pseudomembrane implied a worse prognosis than other types of TA in severely immunocompromised patients [6], while ulcerative TA might be associated with a better clinical course [9]. In a bronchoscopic examination, TA might present diffuse and focal ulcerative form or whitish plaques related to pseudomembranes [7]. In this case, we experienced a pseudomembranous form of TA, which was resistant to an antifungal agent.
The clinical presentation is variable and nonspecific; thus, it is difficult to be diagnosed based on subjective symptoms [2]. Recently, galactomannan polysaccharides have been proposed to diagnose invasive aspergillosis, which demonstrated fair sensitivity (71.5%) and specificity (93.0%) [10]. In addition, serum galactomannan may be related to treatment outcomes of invasive aspergillosis [10]. Therefore, for early diagnosis in case of high suspicion for TA, it should be considered to perform an endoscopic examination and serial measurement of serum galactomannan in patients with neutropenia and immunocompromised status. In this case report, the patient received regular follow-up of serum galactomannan level, which was negative when the patient was asymptomatic, increased with symptoms and decreased after surgery.
The biopsy of the mucosal lesion is a crucial step for proper diagnosis and treatment of TA, which could be performed with bronchoscopy and endobronchial ultrasound-guided transbronchial needle aspiration. Unfortunately, there has been no concrete consensus for the management of TA. Voriconazole has been regarded as the first-line agent for the treatment of invasive aspergillosis but previous studies supported that TA might be improved with systemic antifungal agents combined with surgical intervention [5,11]. Thus surgical intervention should be considered when the patient is resistant to medical treatment, like in our case report. During the surgery we performed end-to-end anastomosis after confirming the viability of the remained tissue, because invasive aspergillosis could cause ischemic injury and wound complication by invasion of blood vessels.
TA could be a life-threatening disease when presenting with massive hemoptysis due to hyphae invasion into the vasculature and acute airway obstruction secondary to accumulation of pseudomembranes [5,11,12]. Therefore, early suspicion and diagnosis of the disease are critical. In case of suspicious TA in high-risk patients, active diagnostic evaluation such as bronchoscopy should be implemented, and a combination of medical and surgical treatment should be considered.
Acknowledgements
None.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Authors’ Contribution
Conceptualization: all authors. Data curation: all authors. Methodology: all authors. Project administration: Keon Hee Yoo, Man Ki Chung. Visualization: Yujin Heo, Nayeon Choi, Man Ki Chung. Writing—original draft: Yujin Heo, Nayeon Choi, Man Ki Chung. Writing—review & editing: Man Ki Chung. Approval of final manuscript: all authors.
