Oncologic Outcomes of T1–T2N0 Glottic Cancer Treatment: Single Center Experiences of 417 Patients Over 20 Years
Article information
Abstract
Background and Objectives
For T1–T2 early glottic cancer, single modality treatment with radiation therapy (RT) or transoral laser microsurgery is the standard therapeutic option. However, the choice between surgery and RT has been debated for decades. Even though patient selection bias for each modality inherently exists in the retrospective study, this study aimed to compare the oncologic outcomes of the actual treatment of these patients between surgery-based treatment and RT.
Materials and Method
The medical records of 417 patients with T1–T2N0 glottic squamous cell carcinoma were reviewed who were treated at our institution between 1995 and 2014. The patients were divided into two groups; primarily surgery-based treatment (OP, n=209) or RT (n=208).
Results
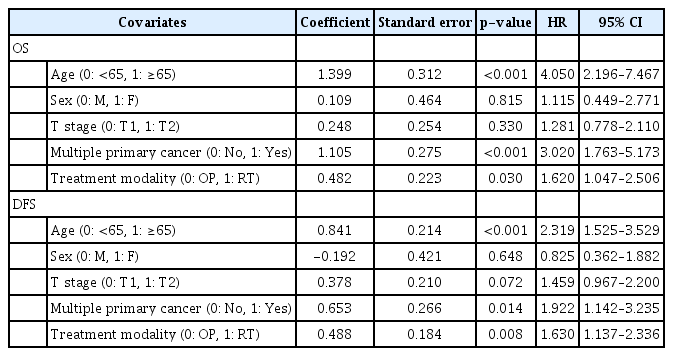
In the T1 stage, local failure, overall survival (OS), and disease-free survival (DFS) rates were not different between the OP and RT groups. However, in the T2 stage, the local failure rate was higher in the RT group (p<0.01). OS and DFS were higher in the OP group (p=0.019 and p=0.004, respectively). Larynx-preservation rate was similar in both groups (97.1% and 96.2%, p=0.576). Multivariate analysis showed that age (>65), presence of multiple primary cancer, and treatment modality were significant variables influencing OS and DFS.
Conclusion
Surgery-based treatment provided better local control rates, DFS, and OS in patients with T1–T2N0 glottic SCC. In the T1 stage, treatment outcomes were similar between OP and RT groups. In the T2 stage, OP showed better results than RT, suggesting that refined strategies are required to improve the oncologic outcomes of RT for T2 glottic cancer.
INTRODUCTION
Laryngeal carcinoma is one of the most common malignancies of the head and neck. The glottis is involved in 51% of laryngeal malignancy, and 80% of glottic cancer is diagnosed in the early stage [1]. For T1–T2 early glottic cancer, single modality treatment with radiation therapy (RT) or surgery (OP), including transoral laser microsurgery (TLM) and, less frequently, open partial laryngectomy (OPL) are the standard therapeutic options [2]. For several decades, the choice between OP and RT has been a continuing debate regarding oncologic and functional outcomes for these patients.
Recent systematic reviews and meta-analysis studies demonstrated that when compared to RT, TLM resulted in higher rates of overall survival and/or laryngeal preservation in patients of T1 [3,4] and T2 stage [5,6], while local control (LC) rates were not different between RT and TLM in T1–T2 stages [3-5,7,8]. For functional outcomes, maximum phonation time was longer in the RT group [4,9]. Self-perception of voice disability was comparable between TLM and RT [4,9,10]. Regarding the quality of life, there were no significant differences between TLM and RT groups [11], and treatment cost was lower in the TLM group [12].
Most previous studies compared the treatment outcomes of TLM alone and RT [3-5]. However, despite the intent for single modality treatment with surgery, about 5%–13% of early glottic cancer patients required adjuvant radiotherapy because of close surgical margins or the extent of diffuse disease [13,14]. Therefore, when analyzing the outcomes of early glottic cancer treatment, surgery-based treatment needs to include cohorts of postoperative adjuvant RT, which may be a more realistic clinical setting. This study aimed to compare the oncologic outcomes of OP and RT for T1–T2 glottic squamous cell carcinoma (SCC) by reviewing a single-institutional database of patients over the last 20 years.
METHODS
Patients and treatment selection
We reviewed the medical records of laryngeal cancer patients treated at our institution between 1995 and 2014. We selected patients diagnosed with T1–T2N0 glottic SCC based on laryngoscopic/stroboscopic findings, contrast-enhanced CT, and laryngomicroscopic evaluation and biopsy. We excluded patients who received primary treatment at other hospitals. Our Institutional Review Board approved this study protocol (IRB No. 2020-07-025).
Patients were divided into two groups according to the primary treatment; the surgery-based treatment group (OP, n=209) and the RT group (RT, n=208). The OP group includes the patients of surgery alone (n=160) and surgery plus adjuvant RT (n=49). Surgery included TLM (n=185) and OPL (n=24). Most of the TLM was performed at the day surgery center with a CO2 laser (Acupulse, Lumenis, Yokeneam, Israel) using the 1–2 W, super-pulse, and continuous modes. Tumors were resected with 2–3 mm peripheral margins. Surgical margins from the remnant mucosa and remaining deep tissues were submitted for frozen biopsy. When a moderate or higher grade of dysplasia was reported from the frozen biopsy, further resection was performed if possible. OPL included supra-cricoid partial laryngectomy (n=6), vertical partial hemi-laryngectomy (n=17), and laryngofissure with cordectomy (n=2).
Two different dose-fractionation schedules were used in the study period for definitive RT. A total dose of 66 to 70 Gy in 2.0 Gy per fraction over seven weeks was used from 1995 to May 2001. After that, a hypo-fractionation schedule was applied. A total dose of 63 to 67.5 Gy in 2.25 Gy per fraction was delivered over six weeks. Conventional planning with a single slice of larynx contour was applied until March 2007. After that, 3D planning with CT simulation was used. The intensity-modulated RT (IMRT) technique has been applied since February 2013.
The decision on treatment modality was mainly made at the tumor board meetings after discussing with the head and neck surgery specialists, radiation oncologists, and the patients. The treatment modality was primarily determined according to the extent of the disease. TLM was preferred as the first treatment of choice except when the patients have bilateral vocal fold lesions or discernable subglottic/supraglottic diffuse extension. Radiotherapy was recommended when the patients were concerned with their voice quality. Limited lesion visualization during laryngomicroscopic evaluation was another reason for RT recommendation. The addition of adjuvant RT after surgery was determined at the tumor-board meetings. All patients were regularly evaluated at 3-to-6-month intervals until at least five years after completion of the primary treatment. Stroboscopic examination and CT scans were alternatively performed during the follow-up evaluations, while basic physical assessment, including a rigid or flexible laryngoscopic analysis, was performed at every visit.
Outcome measurements
To compare the oncologic outcome of each treatment, five-year disease-free survival (DFS) and five-year overall survival (OS) rates were calculated based on the time-to-interval from the primary treatment. The DFS events included both disease recurrence and death. The laryngeal preservation rate was calculated based on whether a total laryngectomy was conducted. For recurring cases, failure patterns were categorized into local, regional, loco-regional, and distant failure categories. Local recurrences were further analyzed according to the stage, the anterior commissure (AC) involvement, types of surgery, and the addition of adjuvant RT.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA), and p-values for each variable were calculated using the chi-square test and Fisher exact test. OS and DFS curves were developed using the Kaplan-Meier survival analysis, and the log-rank test’s differences between the two groups were evaluated. The prognostic significance of variables was assessed by univariable and multivariable analyses using the Cox proportional hazard model. Differences were considered statistically significant at p<0.05.
RESULTS
Patient characteristics
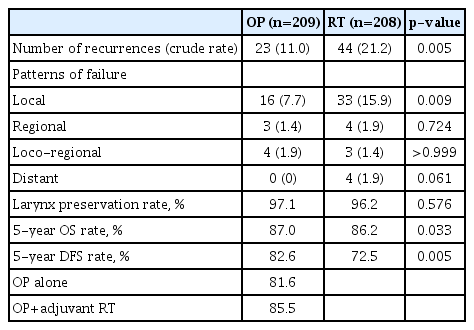
The flow chart of patient enrollment and exclusion details are summarized in Fig. 1. A total of 417 patients with T1–T2N0 glottic SCC were enrolled in this study. The OP group included 209 patients, and the RT group included 208 patients. Patient characteristics are summarized in Table 1. The median follow-up period was 60.5 and 89 months for OP and RT groups, respectively. Mean age, sex distribution, the presence of multiple primary cancers, and the proportion of AC involvement were not different between the two groups. The ratio of T2 (p=0.013) and T1b stages (p=0.012) were higher in the RT group.
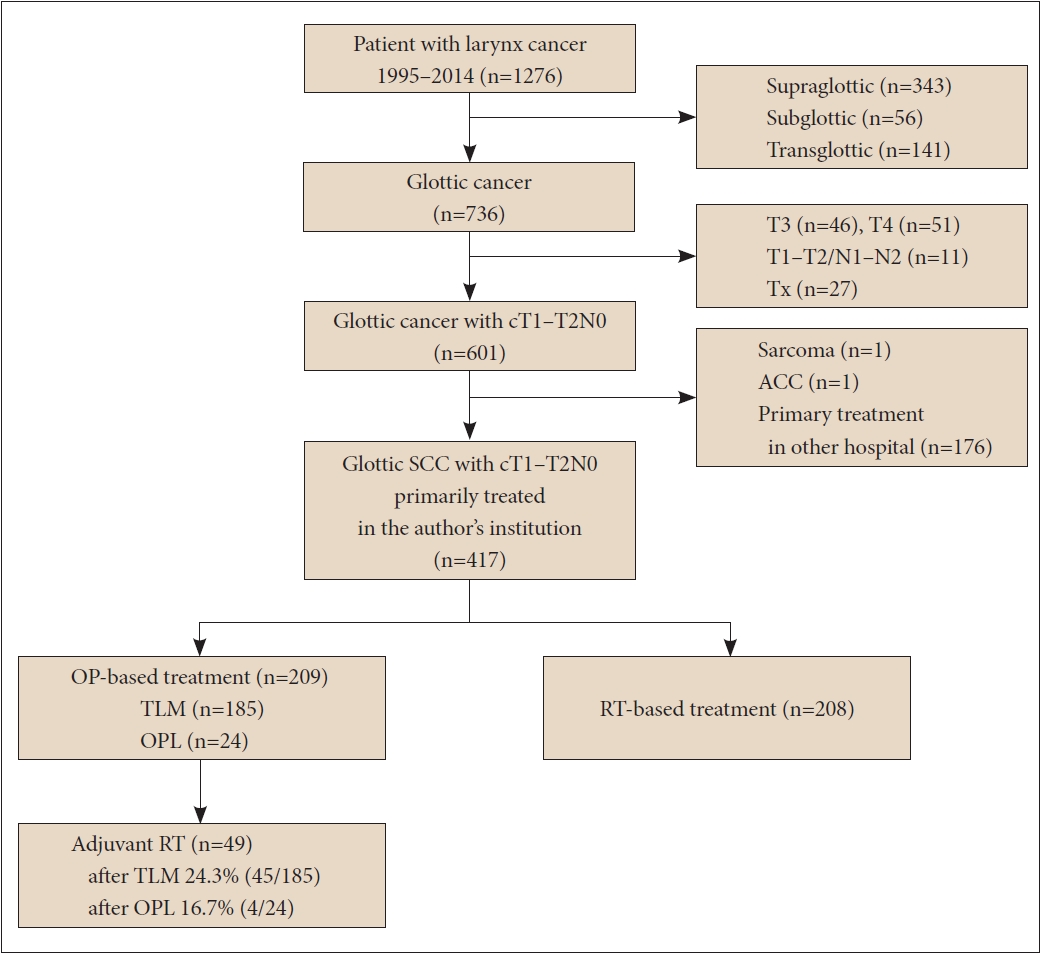
Flow chart of patient selection. SCC, squamous cell carcinoma; TLM, transoral laser microsurgery; OPL, open partial laryngectomy; ACC, adenoid cystic carcinoma; OP, surgery; RT, radiotherapy.
Treatment outcomes
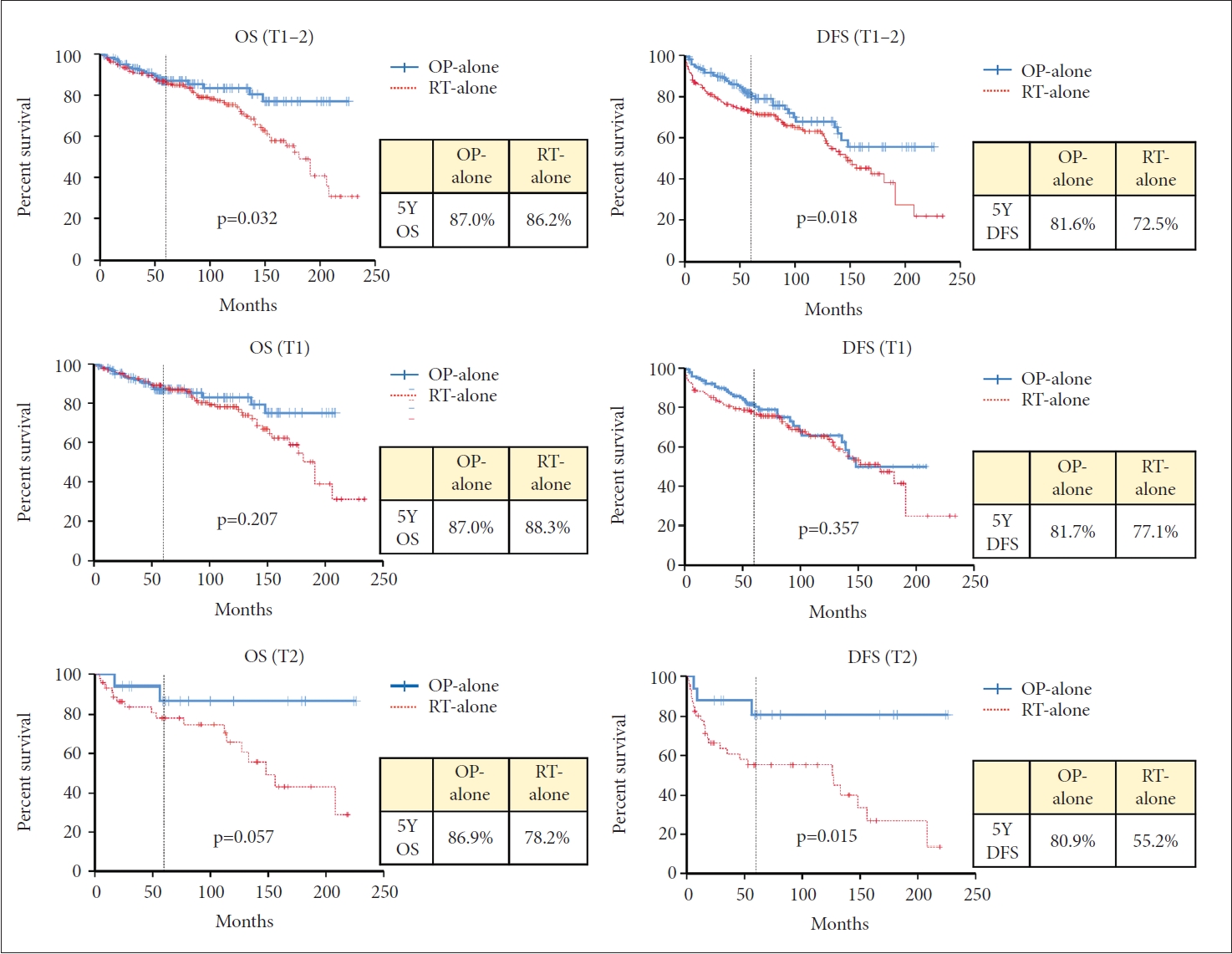
Recurrence was observed in 67 cases after primary treatment; 23 (11.0%) in the OP group and 44 (21.2%) in the RT group (p=0.005) (Table 2). Local recurrence was the most common pattern of failure in both groups. In the OP group, local failure occurred in 16 patients, loco-regional in 4 and regional in 3. In the RT group, local failure occurred in 33 patients, locoregional in 3, regional in 4, and distant in 4. The laryngeal preservation rate was similar in both groups (OP vs. RT, 97.1% and 96.2%, p=0.576). The five-year OS rate was 87.0% and 86.2% for the OP and RT groups, respectively (p=0.033). The five-year DFS rate was 82.6% and 72.5% for each group (p=0.005) (Fig. 2 and Table 2). When OS and DFS rates were compared in T1 and T2 stages separately, both OS and DFS rates were higher in the OP group in the T2 stage (p=0.019 and p=0.004, respectively). In the T1 stage, however, OS and DFS rates were not different between the OP and RT groups (Fig. 2). In multivariate analysis for OS and DFS, age and the presence of multiple primary cancer were significant factors for OS and DFS. A primary treatment modality was also an essential factor for OS and DFS (Table 3).

Five-year overall survival (OS) and disease-free survival (DFS) of OP and RT groups. OP, surgery; RT, radiotherapy.
Analysis of local failures
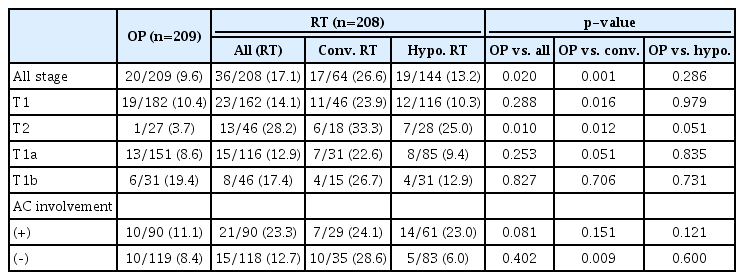
Local failures (including logo-regional failure) were more common in the RT group (Table 4); 20 (9.6%) in the OP group, and 36 (17.1%) in the RT group (p=0.02). In patients with the T1 stage, local failure rates were not different between the OP and RT groups, especially in the RT group treated with a hypo-fractionation schedule. In a subgroup analysis of the T1a and T1b stages, there was no difference in local failure rates between the two groups. However, in the T2 stage, the local failure rate was higher in the RT group (p<0.01), but the difference was marginal in the RT group treated with a hypo-fractionated schedule (p=0.051). When there was AC involvement, local failure tended to be higher in the RT group than in the OP group (11.1% for the OP group and 23.3% for the RT group), though the difference was not statistically significant (p=0.081). Regarding patients with adjuvant RT, there was no difference in the local failure rate; 16 (10.0%) in the OP alone group and 4 (8.2%) in the OP plus adjuvant RT group (p=0.702).
Subgroup analysis of the surgery-based treatment group
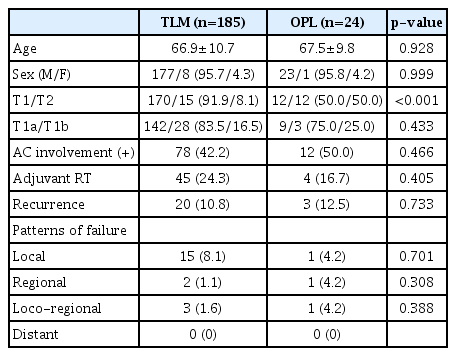
Mean age and sex distribution were not different between the TLM (n=185) and OPL (n=24) groups. The proportion of the T2 stage was higher in the OPL group (8.1% vs. 50.0%, p<0.001). The ratio of T1a to T1b and AC involvement was not different between the two groups. Adjuvant RT was delivered for 45 (24.3%) in the TLM group and 4 (16.7%) in the OPL group (p=0.405). Overall recurrence rate and failure patterns were not different between these two groups (Table 5).
Surgery alone vs. radiation therapy
When patients who received surgery without adjuvant RT (surgery-alone, n=160) were compared to patients who received definitive RT (n=208), the five-year OS rate was 87.0% and 86.2% for the surgery-alone and RT groups, respectively (p=0.032) (Fig. 3). Five-year DFS rate was 81.6% and 72.5% for each group (p=0.018). When the survival rates were compared according to the T stage, the DFS rate for the T2 stage in the surgery-alone group was better than that of the RT group (80.9% and 55.2% at five-year, respectively, p=0.015). OS rate for the T2 stage was slightly better in the surgery-alone group than the RT group (86.9% and 78.2% at five-year, respectively, p=0.057), but without a statistical significance. For the T1 stage, however, there were no differences in DFS or OS rates between the two groups (p=0.357 and p=0.207, respectively) (Fig. 3).
DISCUSSION
This study compared the oncologic outcomes between OP- and RT-based treatments for T1–T2N0 glottic SCC. A recent systematic review and population-based studies showed conflicting results for comparing oncologic outcomes between OP and RT. For LC rates, there was no difference between TLM and RT in the T1 stage [3,8], Tis-T1 stage [4], and Tis-T2 stage [5]. For survival outcomes, OS was superior in TLM for the T1–T2 stage in some studies [5], while there was no difference for the Tis-T1 stage in other studies [4].
In our study, when analyzed in patients with T1–T2 stage together, five-year DFS and OS were better in the OP group than in the RT group. When analyzed separately according to the T stage, the OP group showed better DFS and OS in the T2 stage, but not in the T1 stage (Fig. 2). Also, the OP group showed better LC rates in the T2 stage. The larynx-preservation rate was comparable between the two groups. We also compared the outcomes of the patients with surgery alone to the RT group. When analyzed in patients with the T1–T2 stage together, OS and DFS were better in the surgery-alone group. This trend was only found in the T2 stage when analyzed separately according to the T stage (Fig. 3).
In treating early glottic cancer, it is challenging to compare OP and RT directly as there has been no confirmative randomized trial to compare the two modalities. When retrospectively analyzed, patient selection bias for each modality exists inherently. Therefore, until now, there is no compelling evidence favoring one modality over the other regarding oncologic outcomes. The treatment modality is determined by the extent and visualization of the disease, medical co-morbidities, pre-treatment voice and swallowing functions, physician experience, and physician and patient preferences [4]. Suppose the patient has a well-visualized disease with a limited extent and good performance. In that case, TLM is preferred as the first treatment of choice because of cost, convenience, and good voice quality results. However, in the opposite case, if the functional outcome after resection is expected to be poor because of a more diffuse extent or the patient is medically unfit for general anesthesia, RT is generally recommended. Our study population also showed that the RT group had a higher proportion of T1b and T2 stage disease, which might reflect typical treatment recommendations that most T1b and T2 tumors are harder to resect surgically with negative margins without significant impact on function and, therefore, first-line therapy tends to be radiation. Accordingly, with this selection bias, it should be cautious about concluding that surgery provides better oncologic outcomes than RT in early glottic SCC.
In treating T1–T2 early glottic cancer, a single-modality treatment is generally recommended because the bi-modality treatment is associated with poor functional outcomes, as discussed in the ASCO guidelines [15]. However, adding adjuvant RT is often considered in cases of insufficient surgical margin and/or severe dysplasia around the tumors. Unlike most previous studies, patients who received postoperative RT after curative-intent surgery were included in this study. In addition, the proportion of adjuvant RT in the OP group (23.5%) was higher than that previously reported (5%–13%) [13,14]. Postoperative adjuvant RT was performed in 10 of 27 (37%) patients with T2-stage disease. While the surgical group is confounded by dual-modality therapy, it does reflect the reality of challenges faced by these patients and perhaps better represents the actual treatment of these patients. There was no difference in LC between surgery and surgery with postoperative radiation groups. However, the low number of recurrences does limit the interpretation of the difference in LC between these two surgical groups.
Several factors are known to have negative impacts on LC. In tumor factors, AC involvement and impaired cord mobility of the T2 stage remain controversial. AC involvement may affect LC because of under-staging due to microscopic spread to a cricothyroid membrane, pre-epiglottic space, or para-epiglottic space, the possibility of incomplete resection due to difficulty in surgical exposure, and the underdosing of radiation at the air-tissue interface. One recent meta-analysis study showed that AC involvement decreased the five-year LC rate by 12% in T1 glottic cancer [16]. In the current study, AC involvement affected LC only in the RT group. When AC was involved, the LC rate decreased (23.3% vs. 12.7%) in the RT group, while similar (11.1% vs. 8.4%) in the OP group. One systematic review study noted that the use of binary variables (yes or no) for AC involvement leads to inconsistent results in prognostic significance, where they showed that AC involvement did not have a significant impact on LC in 23 out of 34 (67.6%) studies in the RT group and 18 out of 24 (75.0%) studies of the TLM group [17]. This inconsistent result was due to variations in the definition of AC involvement and the detail of the work-up, such as endoscopic and radiologic evaluation, and the treatment and follow-up policy. They concluded that stratifications according to the extent of AC involvement need to be employed to elucidate the prognostic significance of AC involvement.
T2 stage is also a controversial issue in managing early glottic cancer. It is a heterogeneous group, defined as the tumor extending to other subsites of the larynx and/or impaired vocal cord mobility. Tumor volume, the degree of invasion to other subsites, and the degree of mobility impairment can vary significantly, even in the same T2 stage. Several studies demonstrated that the LC rate was similar to T3 rather than T2 when vocal cord mobility was impaired [6,7,18,19]. However, the current American Joint Committee on Cancer staging system for glottic cancer does not divide the T2 stage into subclassifications. Thus, in the T2 stage, it should be noted that selection bias may affect the treatment outcome more significantly compared to the T1 stage. In our data, the oncologic outcomes of the OP group were comparable between T1 and T2 stages (T1 vs. T2, 92.8% vs. 88.9% in LC rate, 86.9% vs. 88.4% in OS rate, and 82.9% vs. 80.9% in DFS rate, respectively).
In contrast, in the RT group, there was a significant difference in the oncologic outcomes between the T1 and T2 stages (T1 vs. T2, 84.4% vs. 62.4% in LC rate, 88.3% vs. 78.2% in OS rate and 77.1% vs. 55.2% in DFS rate, respectively). Several studies reported that the T2 stage negatively impacted LC in patients treated with definitive RT [20-23]. The LC rate ranged from 60% to 80% in the T2 stage, while it consistently exceeded 90% in the T1 stage [24]. For this reason, there have been efforts to improve the outcome for patients with the T2 stage. Fraction size and overall treatment time of RT are known to be associated with LC rate [21,25,26]. A recent study using the National Cancer Data Base registry data reported that hypo-fractionated RT (2.25 Gy/fraction to 63–65.25 Gy) improved survival compared with conventionally fractionated RT (2.0 Gy/fraction to 66–70 Gy), particularly in T2 disease. Similar to these studies, our data showed that hypo-fractionated RT showed comparable LC and survival outcomes to surgery in the T1 stage, while conventional RT showed worse results. In contrast, hypo-fractionated RT failed to achieve similar treatment outcomes in the OP group in the T2 stages. Several retrospective studies reported that concurrent chemotherapy improved the LC and larynx-preservation rates in early glottic cancer [27-29]. Still, currently, there is a lack of well-designed prospective trials.
The functional outcome, as well as the oncologic outcome, is an essential factor in deciding the treatment modality in early glottic cancer. The laryngeal preservation rate is one of the significant functional outcomes. In several meta-analysis studies, TLM resulted in higher laryngeal preservation rates in patients of the T1 [3,4] and T2 [5,6] stages. In contrast, our data showed no difference in the larynx-preservation rate (97.2% in the OP group vs. 96.7% in the RT group). Voice and swallowing quality are also important functional outcomes. Several studies have shown that TLM resulted in superior voice outcomes to RT [10,30,31]; other studies have reported that voice quality is better after RT than TLM [32,33]. Surgery can preserve the viscoelasticity of the contralateral vocal fold, which is an essential factor for better voice quality, while the extent of resection can affect the quality of voice negatively compared to RT. Recently, one study showed an interesting outcome that single vocal cord irradiation using IMRT improved voice quality compared to conventional techniques [34]. IMRT reduced the RT dose to the contralateral vocal cord in several dosimetric studies [35-37]. In addition, the necessity of adjuvant RT may result in poorer functional outcomes. Jepsen et al. [38] evaluated swallowing and voice outcomes in 17 patients who received adjuvant RT. The Voice Handicap Index and MD Anderson Dysphagia Index were higher in these patients than in patients treated with surgery alone.
There are several significant limitations of this study. Heterogenous treatment types were analyzed together or separately, which could confuse the interpretation of the results; TLM vs. OPL, surgery-alone vs. surgery with adjuvant RT, and conventionally vs. hypo-fractionated RT. The proportion of more extended tumors as T2 and T1b were higher in the RT group. Functional outcomes, including voice and dysphagia quality, were not analyzed. Although this study included a relatively large number of patients over a long period, it was a retrospective study and examined single institutional experiences. Therefore, there will be a limitation in generalizing the findings obtained from this study.
In conclusion, for treating T1–T2 early glottic cancer, the choice between OP and RT has been debated for decades. According to this study, surgery-based treatment provided better OS and DFS than RT in early-stage glottic cancers. Especially in the T2 stage, OP showed better outcomes than RT, suggesting that refined strategies are required to improve the oncologic outcomes of RT for T2 glottic cancer.
Acknowledgements
None
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Authors’ Contribution
Conceptualization: Yong Chan Ahn, Young-Ik Son. Data curation: Sung Young Choi, Man Ki Chung, Dongryul Oh. Formal analysis: Sung Young Choi, Man Ki Chung, Dongryul Oh. Methodology: Sung Young Choi, Man Ki Chung. Project administration: Sung Young Choi, Man Ki Chung. Writing—original draft: Sung Young Choi, Dongryul Oh. Writing—review & editing: Yong Chan Ahn, Young-Ik Son. Approval of final manuscript: all authors.