AbstractBackground and ObjectivesFor T1–T2 early glottic cancer, single modality treatment with radiation therapy (RT) or transoral laser microsurgery is the standard therapeutic option. However, the choice between surgery and RT has been debated for decades. Even though patient selection bias for each modality inherently exists in the retrospective study, this study aimed to compare the oncologic outcomes of the actual treatment of these patients between surgery-based treatment and RT.
Materials and MethodThe medical records of 417 patients with T1–T2N0 glottic squamous cell carcinoma were reviewed who were treated at our institution between 1995 and 2014. The patients were divided into two groups; primarily surgery-based treatment (OP, n=209) or RT (n=208).
ResultsIn the T1 stage, local failure, overall survival (OS), and disease-free survival (DFS) rates were not different between the OP and RT groups. However, in the T2 stage, the local failure rate was higher in the RT group (p<0.01). OS and DFS were higher in the OP group (p=0.019 and p=0.004, respectively). Larynx-preservation rate was similar in both groups (97.1% and 96.2%, p=0.576). Multivariate analysis showed that age (>65), presence of multiple primary cancer, and treatment modality were significant variables influencing OS and DFS.
ConclusionSurgery-based treatment provided better local control rates, DFS, and OS in patients with T1–T2N0 glottic SCC. In the T1 stage, treatment outcomes were similar between OP and RT groups. In the T2 stage, OP showed better results than RT, suggesting that refined strategies are required to improve the oncologic outcomes of RT for T2 glottic cancer.
INTRODUCTIONLaryngeal carcinoma is one of the most common malignancies of the head and neck. The glottis is involved in 51% of laryngeal malignancy, and 80% of glottic cancer is diagnosed in the early stage [1]. For T1–T2 early glottic cancer, single modality treatment with radiation therapy (RT) or surgery (OP), including transoral laser microsurgery (TLM) and, less frequently, open partial laryngectomy (OPL) are the standard therapeutic options [2]. For several decades, the choice between OP and RT has been a continuing debate regarding oncologic and functional outcomes for these patients.
Recent systematic reviews and meta-analysis studies demonstrated that when compared to RT, TLM resulted in higher rates of overall survival and/or laryngeal preservation in patients of T1 [3,4] and T2 stage [5,6], while local control (LC) rates were not different between RT and TLM in T1–T2 stages [3-5,7,8]. For functional outcomes, maximum phonation time was longer in the RT group [4,9]. Self-perception of voice disability was comparable between TLM and RT [4,9,10]. Regarding the quality of life, there were no significant differences between TLM and RT groups [11], and treatment cost was lower in the TLM group [12].
Most previous studies compared the treatment outcomes of TLM alone and RT [3-5]. However, despite the intent for single modality treatment with surgery, about 5%–13% of early glottic cancer patients required adjuvant radiotherapy because of close surgical margins or the extent of diffuse disease [13,14]. Therefore, when analyzing the outcomes of early glottic cancer treatment, surgery-based treatment needs to include cohorts of postoperative adjuvant RT, which may be a more realistic clinical setting. This study aimed to compare the oncologic outcomes of OP and RT for T1–T2 glottic squamous cell carcinoma (SCC) by reviewing a single-institutional database of patients over the last 20 years.
METHODSPatients and treatment selectionWe reviewed the medical records of laryngeal cancer patients treated at our institution between 1995 and 2014. We selected patients diagnosed with T1–T2N0 glottic SCC based on laryngoscopic/stroboscopic findings, contrast-enhanced CT, and laryngomicroscopic evaluation and biopsy. We excluded patients who received primary treatment at other hospitals. Our Institutional Review Board approved this study protocol (IRB No. 2020-07-025).
Patients were divided into two groups according to the primary treatment; the surgery-based treatment group (OP, n=209) and the RT group (RT, n=208). The OP group includes the patients of surgery alone (n=160) and surgery plus adjuvant RT (n=49). Surgery included TLM (n=185) and OPL (n=24). Most of the TLM was performed at the day surgery center with a CO2 laser (Acupulse, Lumenis, Yokeneam, Israel) using the 1–2 W, super-pulse, and continuous modes. Tumors were resected with 2–3 mm peripheral margins. Surgical margins from the remnant mucosa and remaining deep tissues were submitted for frozen biopsy. When a moderate or higher grade of dysplasia was reported from the frozen biopsy, further resection was performed if possible. OPL included supra-cricoid partial laryngectomy (n=6), vertical partial hemi-laryngectomy (n=17), and laryngofissure with cordectomy (n=2).
Two different dose-fractionation schedules were used in the study period for definitive RT. A total dose of 66 to 70 Gy in 2.0 Gy per fraction over seven weeks was used from 1995 to May 2001. After that, a hypo-fractionation schedule was applied. A total dose of 63 to 67.5 Gy in 2.25 Gy per fraction was delivered over six weeks. Conventional planning with a single slice of larynx contour was applied until March 2007. After that, 3D planning with CT simulation was used. The intensity-modulated RT (IMRT) technique has been applied since February 2013.
The decision on treatment modality was mainly made at the tumor board meetings after discussing with the head and neck surgery specialists, radiation oncologists, and the patients. The treatment modality was primarily determined according to the extent of the disease. TLM was preferred as the first treatment of choice except when the patients have bilateral vocal fold lesions or discernable subglottic/supraglottic diffuse extension. Radiotherapy was recommended when the patients were concerned with their voice quality. Limited lesion visualization during laryngomicroscopic evaluation was another reason for RT recommendation. The addition of adjuvant RT after surgery was determined at the tumor-board meetings. All patients were regularly evaluated at 3-to-6-month intervals until at least five years after completion of the primary treatment. Stroboscopic examination and CT scans were alternatively performed during the follow-up evaluations, while basic physical assessment, including a rigid or flexible laryngoscopic analysis, was performed at every visit.
Outcome measurementsTo compare the oncologic outcome of each treatment, five-year disease-free survival (DFS) and five-year overall survival (OS) rates were calculated based on the time-to-interval from the primary treatment. The DFS events included both disease recurrence and death. The laryngeal preservation rate was calculated based on whether a total laryngectomy was conducted. For recurring cases, failure patterns were categorized into local, regional, loco-regional, and distant failure categories. Local recurrences were further analyzed according to the stage, the anterior commissure (AC) involvement, types of surgery, and the addition of adjuvant RT.
Statistical analysisStatistical analysis was performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA), and p-values for each variable were calculated using the chi-square test and Fisher exact test. OS and DFS curves were developed using the Kaplan-Meier survival analysis, and the log-rank test’s differences between the two groups were evaluated. The prognostic significance of variables was assessed by univariable and multivariable analyses using the Cox proportional hazard model. Differences were considered statistically significant at p<0.05.
RESULTSPatient characteristicsThe flow chart of patient enrollment and exclusion details are summarized in Fig. 1. A total of 417 patients with T1–T2N0 glottic SCC were enrolled in this study. The OP group included 209 patients, and the RT group included 208 patients. Patient characteristics are summarized in Table 1. The median follow-up period was 60.5 and 89 months for OP and RT groups, respectively. Mean age, sex distribution, the presence of multiple primary cancers, and the proportion of AC involvement were not different between the two groups. The ratio of T2 (p=0.013) and T1b stages (p=0.012) were higher in the RT group.
Treatment outcomesRecurrence was observed in 67 cases after primary treatment; 23 (11.0%) in the OP group and 44 (21.2%) in the RT group (p=0.005) (Table 2). Local recurrence was the most common pattern of failure in both groups. In the OP group, local failure occurred in 16 patients, loco-regional in 4 and regional in 3. In the RT group, local failure occurred in 33 patients, locoregional in 3, regional in 4, and distant in 4. The laryngeal preservation rate was similar in both groups (OP vs. RT, 97.1% and 96.2%, p=0.576). The five-year OS rate was 87.0% and 86.2% for the OP and RT groups, respectively (p=0.033). The five-year DFS rate was 82.6% and 72.5% for each group (p=0.005) (Fig. 2 and Table 2). When OS and DFS rates were compared in T1 and T2 stages separately, both OS and DFS rates were higher in the OP group in the T2 stage (p=0.019 and p=0.004, respectively). In the T1 stage, however, OS and DFS rates were not different between the OP and RT groups (Fig. 2). In multivariate analysis for OS and DFS, age and the presence of multiple primary cancer were significant factors for OS and DFS. A primary treatment modality was also an essential factor for OS and DFS (Table 3).
Analysis of local failuresLocal failures (including logo-regional failure) were more common in the RT group (Table 4); 20 (9.6%) in the OP group, and 36 (17.1%) in the RT group (p=0.02). In patients with the T1 stage, local failure rates were not different between the OP and RT groups, especially in the RT group treated with a hypo-fractionation schedule. In a subgroup analysis of the T1a and T1b stages, there was no difference in local failure rates between the two groups. However, in the T2 stage, the local failure rate was higher in the RT group (p<0.01), but the difference was marginal in the RT group treated with a hypo-fractionated schedule (p=0.051). When there was AC involvement, local failure tended to be higher in the RT group than in the OP group (11.1% for the OP group and 23.3% for the RT group), though the difference was not statistically significant (p=0.081). Regarding patients with adjuvant RT, there was no difference in the local failure rate; 16 (10.0%) in the OP alone group and 4 (8.2%) in the OP plus adjuvant RT group (p=0.702).
Subgroup analysis of the surgery-based treatment groupMean age and sex distribution were not different between the TLM (n=185) and OPL (n=24) groups. The proportion of the T2 stage was higher in the OPL group (8.1% vs. 50.0%, p<0.001). The ratio of T1a to T1b and AC involvement was not different between the two groups. Adjuvant RT was delivered for 45 (24.3%) in the TLM group and 4 (16.7%) in the OPL group (p=0.405). Overall recurrence rate and failure patterns were not different between these two groups (Table 5).
Surgery alone vs. radiation therapyWhen patients who received surgery without adjuvant RT (surgery-alone, n=160) were compared to patients who received definitive RT (n=208), the five-year OS rate was 87.0% and 86.2% for the surgery-alone and RT groups, respectively (p=0.032) (Fig. 3). Five-year DFS rate was 81.6% and 72.5% for each group (p=0.018). When the survival rates were compared according to the T stage, the DFS rate for the T2 stage in the surgery-alone group was better than that of the RT group (80.9% and 55.2% at five-year, respectively, p=0.015). OS rate for the T2 stage was slightly better in the surgery-alone group than the RT group (86.9% and 78.2% at five-year, respectively, p=0.057), but without a statistical significance. For the T1 stage, however, there were no differences in DFS or OS rates between the two groups (p=0.357 and p=0.207, respectively) (Fig. 3).
DISCUSSIONThis study compared the oncologic outcomes between OP- and RT-based treatments for T1–T2N0 glottic SCC. A recent systematic review and population-based studies showed conflicting results for comparing oncologic outcomes between OP and RT. For LC rates, there was no difference between TLM and RT in the T1 stage [3,8], Tis-T1 stage [4], and Tis-T2 stage [5]. For survival outcomes, OS was superior in TLM for the T1–T2 stage in some studies [5], while there was no difference for the Tis-T1 stage in other studies [4].
In our study, when analyzed in patients with T1–T2 stage together, five-year DFS and OS were better in the OP group than in the RT group. When analyzed separately according to the T stage, the OP group showed better DFS and OS in the T2 stage, but not in the T1 stage (Fig. 2). Also, the OP group showed better LC rates in the T2 stage. The larynx-preservation rate was comparable between the two groups. We also compared the outcomes of the patients with surgery alone to the RT group. When analyzed in patients with the T1–T2 stage together, OS and DFS were better in the surgery-alone group. This trend was only found in the T2 stage when analyzed separately according to the T stage (Fig. 3).
In treating early glottic cancer, it is challenging to compare OP and RT directly as there has been no confirmative randomized trial to compare the two modalities. When retrospectively analyzed, patient selection bias for each modality exists inherently. Therefore, until now, there is no compelling evidence favoring one modality over the other regarding oncologic outcomes. The treatment modality is determined by the extent and visualization of the disease, medical co-morbidities, pre-treatment voice and swallowing functions, physician experience, and physician and patient preferences [4]. Suppose the patient has a well-visualized disease with a limited extent and good performance. In that case, TLM is preferred as the first treatment of choice because of cost, convenience, and good voice quality results. However, in the opposite case, if the functional outcome after resection is expected to be poor because of a more diffuse extent or the patient is medically unfit for general anesthesia, RT is generally recommended. Our study population also showed that the RT group had a higher proportion of T1b and T2 stage disease, which might reflect typical treatment recommendations that most T1b and T2 tumors are harder to resect surgically with negative margins without significant impact on function and, therefore, first-line therapy tends to be radiation. Accordingly, with this selection bias, it should be cautious about concluding that surgery provides better oncologic outcomes than RT in early glottic SCC.
In treating T1–T2 early glottic cancer, a single-modality treatment is generally recommended because the bi-modality treatment is associated with poor functional outcomes, as discussed in the ASCO guidelines [15]. However, adding adjuvant RT is often considered in cases of insufficient surgical margin and/or severe dysplasia around the tumors. Unlike most previous studies, patients who received postoperative RT after curative-intent surgery were included in this study. In addition, the proportion of adjuvant RT in the OP group (23.5%) was higher than that previously reported (5%–13%) [13,14]. Postoperative adjuvant RT was performed in 10 of 27 (37%) patients with T2-stage disease. While the surgical group is confounded by dual-modality therapy, it does reflect the reality of challenges faced by these patients and perhaps better represents the actual treatment of these patients. There was no difference in LC between surgery and surgery with postoperative radiation groups. However, the low number of recurrences does limit the interpretation of the difference in LC between these two surgical groups.
Several factors are known to have negative impacts on LC. In tumor factors, AC involvement and impaired cord mobility of the T2 stage remain controversial. AC involvement may affect LC because of under-staging due to microscopic spread to a cricothyroid membrane, pre-epiglottic space, or para-epiglottic space, the possibility of incomplete resection due to difficulty in surgical exposure, and the underdosing of radiation at the air-tissue interface. One recent meta-analysis study showed that AC involvement decreased the five-year LC rate by 12% in T1 glottic cancer [16]. In the current study, AC involvement affected LC only in the RT group. When AC was involved, the LC rate decreased (23.3% vs. 12.7%) in the RT group, while similar (11.1% vs. 8.4%) in the OP group. One systematic review study noted that the use of binary variables (yes or no) for AC involvement leads to inconsistent results in prognostic significance, where they showed that AC involvement did not have a significant impact on LC in 23 out of 34 (67.6%) studies in the RT group and 18 out of 24 (75.0%) studies of the TLM group [17]. This inconsistent result was due to variations in the definition of AC involvement and the detail of the work-up, such as endoscopic and radiologic evaluation, and the treatment and follow-up policy. They concluded that stratifications according to the extent of AC involvement need to be employed to elucidate the prognostic significance of AC involvement.
T2 stage is also a controversial issue in managing early glottic cancer. It is a heterogeneous group, defined as the tumor extending to other subsites of the larynx and/or impaired vocal cord mobility. Tumor volume, the degree of invasion to other subsites, and the degree of mobility impairment can vary significantly, even in the same T2 stage. Several studies demonstrated that the LC rate was similar to T3 rather than T2 when vocal cord mobility was impaired [6,7,18,19]. However, the current American Joint Committee on Cancer staging system for glottic cancer does not divide the T2 stage into subclassifications. Thus, in the T2 stage, it should be noted that selection bias may affect the treatment outcome more significantly compared to the T1 stage. In our data, the oncologic outcomes of the OP group were comparable between T1 and T2 stages (T1 vs. T2, 92.8% vs. 88.9% in LC rate, 86.9% vs. 88.4% in OS rate, and 82.9% vs. 80.9% in DFS rate, respectively).
In contrast, in the RT group, there was a significant difference in the oncologic outcomes between the T1 and T2 stages (T1 vs. T2, 84.4% vs. 62.4% in LC rate, 88.3% vs. 78.2% in OS rate and 77.1% vs. 55.2% in DFS rate, respectively). Several studies reported that the T2 stage negatively impacted LC in patients treated with definitive RT [20-23]. The LC rate ranged from 60% to 80% in the T2 stage, while it consistently exceeded 90% in the T1 stage [24]. For this reason, there have been efforts to improve the outcome for patients with the T2 stage. Fraction size and overall treatment time of RT are known to be associated with LC rate [21,25,26]. A recent study using the National Cancer Data Base registry data reported that hypo-fractionated RT (2.25 Gy/fraction to 63–65.25 Gy) improved survival compared with conventionally fractionated RT (2.0 Gy/fraction to 66–70 Gy), particularly in T2 disease. Similar to these studies, our data showed that hypo-fractionated RT showed comparable LC and survival outcomes to surgery in the T1 stage, while conventional RT showed worse results. In contrast, hypo-fractionated RT failed to achieve similar treatment outcomes in the OP group in the T2 stages. Several retrospective studies reported that concurrent chemotherapy improved the LC and larynx-preservation rates in early glottic cancer [27-29]. Still, currently, there is a lack of well-designed prospective trials.
The functional outcome, as well as the oncologic outcome, is an essential factor in deciding the treatment modality in early glottic cancer. The laryngeal preservation rate is one of the significant functional outcomes. In several meta-analysis studies, TLM resulted in higher laryngeal preservation rates in patients of the T1 [3,4] and T2 [5,6] stages. In contrast, our data showed no difference in the larynx-preservation rate (97.2% in the OP group vs. 96.7% in the RT group). Voice and swallowing quality are also important functional outcomes. Several studies have shown that TLM resulted in superior voice outcomes to RT [10,30,31]; other studies have reported that voice quality is better after RT than TLM [32,33]. Surgery can preserve the viscoelasticity of the contralateral vocal fold, which is an essential factor for better voice quality, while the extent of resection can affect the quality of voice negatively compared to RT. Recently, one study showed an interesting outcome that single vocal cord irradiation using IMRT improved voice quality compared to conventional techniques [34]. IMRT reduced the RT dose to the contralateral vocal cord in several dosimetric studies [35-37]. In addition, the necessity of adjuvant RT may result in poorer functional outcomes. Jepsen et al. [38] evaluated swallowing and voice outcomes in 17 patients who received adjuvant RT. The Voice Handicap Index and MD Anderson Dysphagia Index were higher in these patients than in patients treated with surgery alone.
There are several significant limitations of this study. Heterogenous treatment types were analyzed together or separately, which could confuse the interpretation of the results; TLM vs. OPL, surgery-alone vs. surgery with adjuvant RT, and conventionally vs. hypo-fractionated RT. The proportion of more extended tumors as T2 and T1b were higher in the RT group. Functional outcomes, including voice and dysphagia quality, were not analyzed. Although this study included a relatively large number of patients over a long period, it was a retrospective study and examined single institutional experiences. Therefore, there will be a limitation in generalizing the findings obtained from this study.
In conclusion, for treating T1–T2 early glottic cancer, the choice between OP and RT has been debated for decades. According to this study, surgery-based treatment provided better OS and DFS than RT in early-stage glottic cancers. Especially in the T2 stage, OP showed better outcomes than RT, suggesting that refined strategies are required to improve the oncologic outcomes of RT for T2 glottic cancer.
NOTESAuthors’ Contribution Conceptualization: Yong Chan Ahn, Young-Ik Son. Data curation: Sung Young Choi, Man Ki Chung, Dongryul Oh. Formal analysis: Sung Young Choi, Man Ki Chung, Dongryul Oh. Methodology: Sung Young Choi, Man Ki Chung. Project administration: Sung Young Choi, Man Ki Chung. Writing—original draft: Sung Young Choi, Dongryul Oh. Writing—review & editing: Yong Chan Ahn, Young-Ik Son. Approval of final manuscript: all authors. Fig. 1.Flow chart of patient selection. SCC, squamous cell carcinoma; TLM, transoral laser microsurgery; OPL, open partial laryngectomy;
ACC, adenoid cystic carcinoma; OP, surgery; RT, radiotherapy. 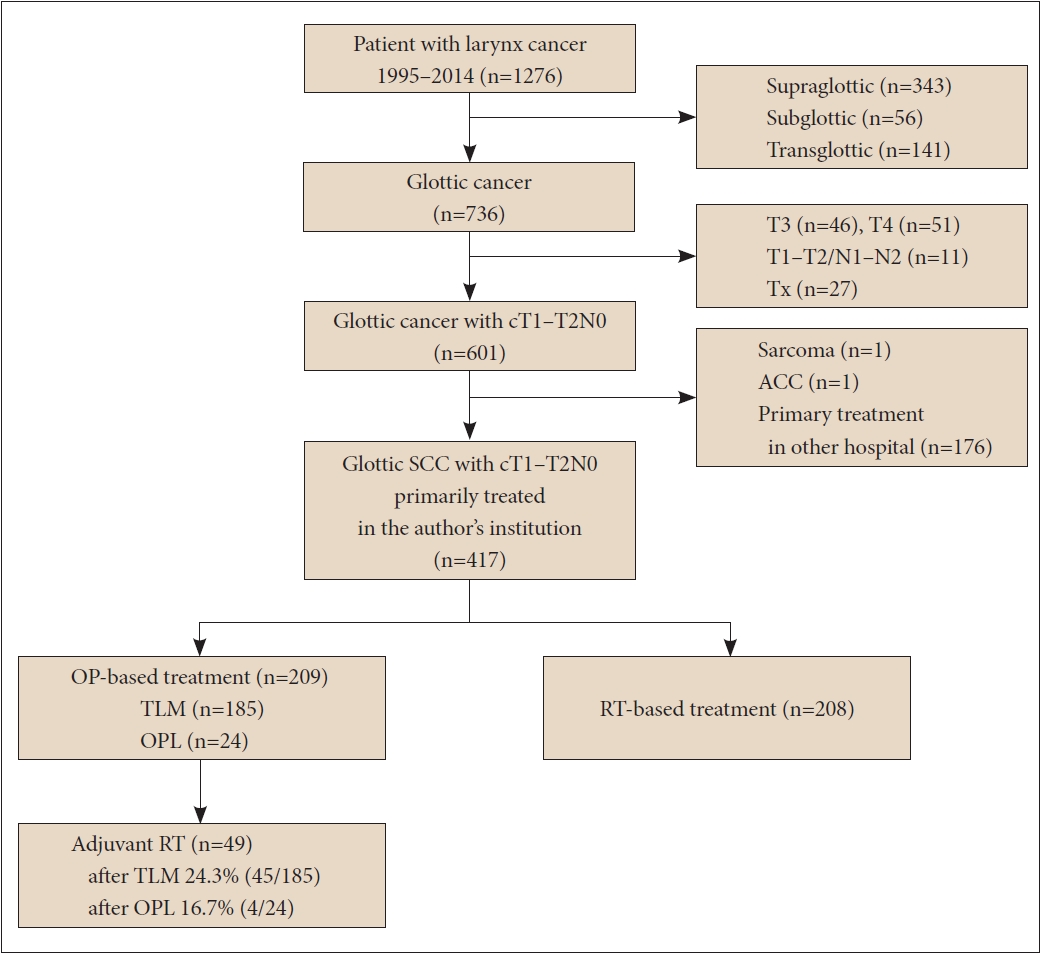
Fig. 2.Five-year overall survival (OS) and disease-free survival (DFS) of OP and RT groups. OP, surgery; RT, radiotherapy. 
Fig. 3.Five-year overall survival (OS) and disease-free survival (DFS) of surgery alone and RT group. OP, surgery; RT, radiotherapy. 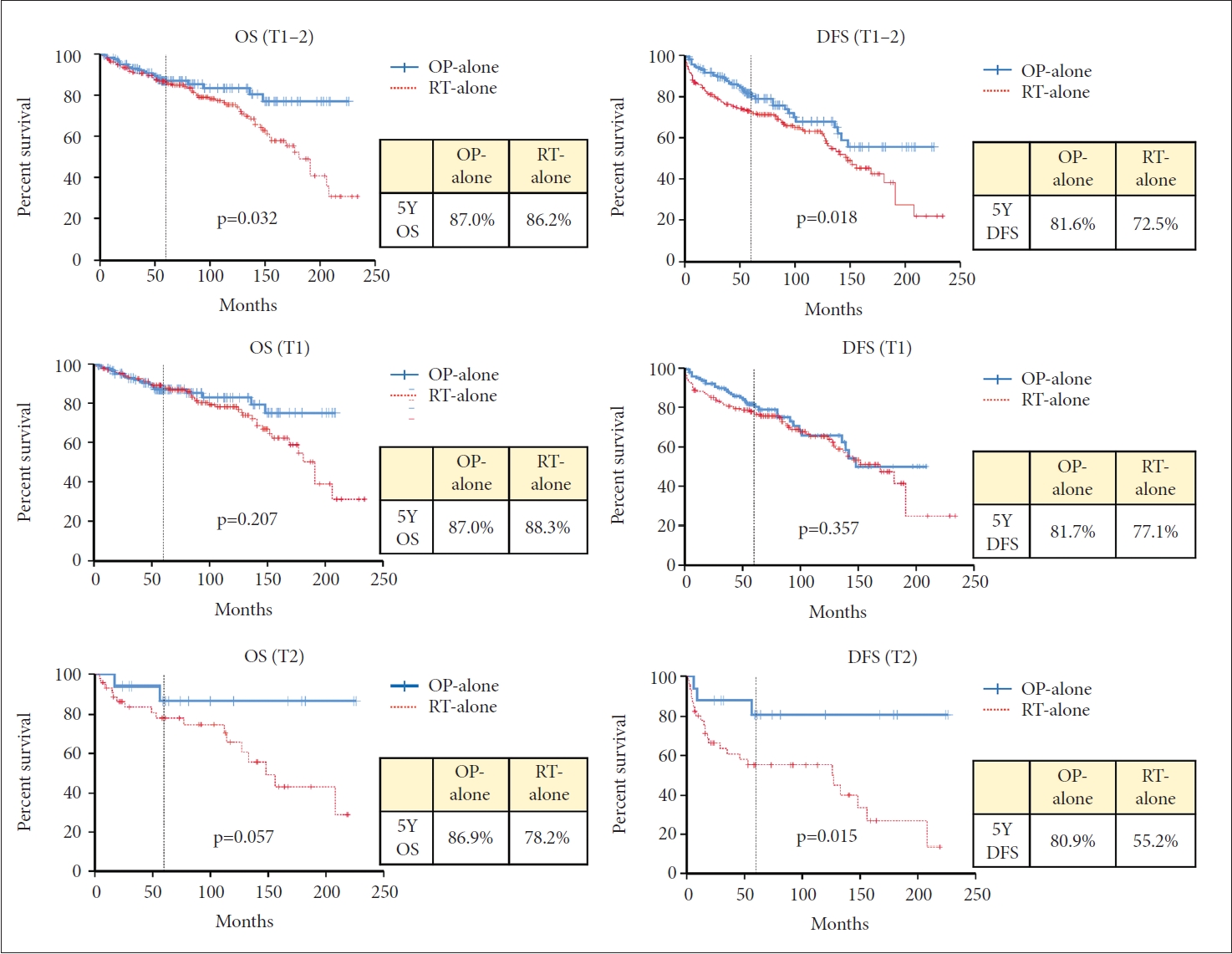
Table 1.Baseline characteristics of the patients in OP and RT group Table 2.Summary of treatment outcomes Table 3.Multivariable analysis for OS and DFS Table 4.Subgroup analysis of local recurrences Table 5.Subgroup analysis of surgery-based treatment group REFERENCES1. Mastronikolis NS, Papadas TA, Goumas PD, Triantaphyllidou IE, Theocharis DA, Papageorgakopoulou N, et al. Head and neck: laryngeal tumors: an overview. Atlas Genet Cytogenet Oncol Haematol 2009;13(11):888-93.
2. Baird BJ, Sung CK, Beadle BM, Divi V. Treatment of early-stage laryngeal cancer: a comparison of treatment options. Oral Oncol 2018;87:8-16.
3. Vaculik MF, MacKay CA, Taylor SM, Trites JRB, Hart RD, Rigby MH. Systematic review and meta-analysis of T1 glottic cancer outcomes comparing CO2 transoral laser microsurgery and radiotherapy. J Otolaryngol Head Neck Surg 2019;48(1):44.
4. Guimarães AV, Dedivitis RA, Matos LL, Aires FT, Cernea CR. Comparison between transoral laser surgery and radiotherapy in the treatment of early glottic cancer: a systematic review and meta-analysis. Sci Rep 2018;8(1):11900.
5. Ding Y, Wang B. Efficacy of laser surgery versus radiotherapy for treatment of glottic carcinoma: a systematic review and meta-analysis. Lasers Med Sci 2019;34(5):847-54.
6. Hendriksma M, Heijnen BJ, Sjögren EV. Oncologic and functional outcomes of patients treated with transoral CO2 laser microsurgery or radiotherapy for T2 glottic carcinoma: a systematic review of the literature. Curr Opin Otolaryngol Head Neck Surg 2018;26(2):84-93.
7. Warner L, Lee K, Homer JJ. Transoral laser microsurgery versus radiotherapy for T2 glottic squamous cell carcinoma: a systematic review of local control outcomes. Clin Otolaryngol 2017;42(3):629-36.
8. Mo HL, Li J, Yang X, Zhang F, Xiong JW, Yang ZL, et al. Transoral laser microsurgery versus radiotherapy for T1 glottic carcinoma: a systematic review and meta-analysis. Lasers Med Sci 2017;32(2):461-7.
9. Huang G, Luo M, Zhang J, Liu H. The voice quality after laser surgery versus radiotherapy of T1a glottic carcinoma: systematic review and meta-analysis. Onco Targets Ther 2017;10:2403-10.
10. Du G, Liu C, Yu W, Li J, Li W, Wang C, et al. Voice outcomes after laser surgery vs. radiotherapy of early glottic carcinoma: a meta-analysis. Int J Clin Exp Med 2015;8(10):17206-13.
11. Spielmann PM, Majumdar S, Morton RP. Quality of life and functional outcomes in the management of early glottic carcinoma: a systematic review of studies comparing radiotherapy and transoral laser microsurgery. Clin Otolaryngol 2010;35(5):373-82.
12. Feng Y, Wang B, Wen S. Laser surgery versus radiotherapy for T1-T2N0 glottic cancer: a meta-analysis. ORL J Otorhinolaryngol Relat Spec 2011;73(6):336-42.
13. Ansarin M, Santoro L, Cattaneo A, Massaro MA, Calabrese L, Giugliano G, et al. Laser surgery for early glottic cancer: impact of margin status on local control and organ preservation. Arch Otolaryngol Head Neck Surg 2009;135(4):385-90.
14. Gallo A, de Vincentiis M, Manciocco V, Simonelli M, Fiorella ML, Shah JP. CO2 laser cordectomy for early-stage glottic carcinoma: a long-term follow-up of 156 cases. Laryngoscope 2002;112(2):370-4.
15. Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2018;36(11):1143-69.
16. Tulli M, Re M, Bondi S, Ferrante L, Dajko M, Giordano L, et al. The prognostic value of anterior commissure involvement in T1 glottic cancer: a systematic review and meta-analysis. Laryngoscope 2020;130(8):1932-40.
17. Hendriksma M, Sjögren EV. Involvement of the anterior commissure in early glottic cancer (Tis-T2): a review of the literature. Cancers (Basel) 2019;11(9):1234.
18. Forner D, Rigby MH, Hart RD, Trites JR, Taylor SM. Oncological and functional outcomes following transoral laser microsurgery in patients with T2a vs T2b glottic squamous cell carcinoma. J Otolaryngol Head Neck Surg 2019;48(1):27.
19. McCoul ED, Har-El G. Meta-analysis of impaired vocal cord mobility as a prognostic factor in T2 glottic carcinoma. Arch Otolaryngol Head Neck Surg 2009;135(5):479-86.
20. Nomura T, Ishikawa J, Ohki M, Ohata A, Araki R, Kikuchi S. Multifactorial analysis of local control and survival in patients with early glottic cancer. Laryngoscope 2020;130(7):1701-6.
21. Le QT, Fu KK, Kroll S, Ryu JK, Quivey JM, Meyler TS, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys 1997;39(1):115-26.
22. Eskiizmir G, Baskın Y, Yalçın F, Ellidokuz H, Ferris RL. Risk factors for radiation failure in early-stage glottic carcinoma: a systematic review and meta-analysis. Oral Oncol 2016;62:90-100.
23. Al-Mamgani A, van Rooij PH, Woutersen DP, Mehilal R, Tans L, Monserez D, et al. Radiotherapy for T1-2N0 glottic cancer: a multivariate analysis of predictive factors for the long-term outcome in 1050 patients and a prospective assessment of quality of life and voice handicap index in a subset of 233 patients. Clin Otolaryngol 2013;38(4):306-12.
24. Chera BS, Amdur RJ, Morris CG, Kirwan JM, Mendenhall WM. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys 2010;78(2):461-6.
25. Sapienza LG, Ning MS, Taguchi S, Calsavara VF, Pellizzon ACA, Gomes MJL, et al. Altered-fractionation radiotherapy improves local control in early-stage glottic carcinoma: a systematic review and meta-analysis of 1762 patients. Oral Oncol 2019;93:8-14.
26. Moon SH, Cho KH, Chung EJ, Lee CG, Lee KC, Chai GY, et al. A prospective randomized trial comparing hypofractionation with conventional fractionation radiotherapy for T1-2 glottic squamous cell carcinomas: results of a Korean Radiation Oncology Group (KROG-0201) study. Radiother Oncol 2014;110(1):98-103.
27. Akimoto T, Nonaka T, Kitamoto Y, Ishikawa H, Ninomiya H, Chikamatsu K, et al. Radiation therapy for T2N0 laryngeal cancer: a retrospective analysis for the impact of concurrent chemotherapy on local control. Int J Radiat Oncol Biol Phys 2006;64(4):995-1001.
28. Kumamoto Y, Masuda M, Kuratomi Y, Toh S, Shinokuma A, Chujo K, et al. “FAR” chemoradiotherapy improves laryngeal preservation rates in patients with T2N0 glottic carcinoma. Head Neck 2002;24(7):637-42.
29. Kitani Y, Kubota A, Furukawa M, Sato K. Prognostic factors for local control in patients receiving radiation therapy for early glottic cancer: anterior commissure involvement and effect of chemoradiotherapy. Eur Arch Otorhinolaryngol 2016;273(4):1011-7.
30. Ma Y, Green R, Pan S, McCabe D, Goldberg L, Woo P. Long-term voice outcome following radiation versus laser microsurgery in early glottic cancer. J Voice 2019;33(2):176-82.
31. Gandhi S, Gupta S, Rajopadhye G. A comparison of phonatory outcome between trans-oral CO2 laser cordectomy and radiotherapy in T1 glottic cancer. Eur Arch Otorhinolaryngol 2018;275(11):2783-6.
32. Kerr P, Mark Taylor S, Rigby M, Myers C, Osborn H, Lambert P, et al. Oncologic and voice outcomes after treatment of early glottic cancer: transoral laser microsurgery versus radiotherapy. J Otolaryngol Head Neck Surg 2012;41(6):381-8.
33. Lee SH, Hong KH, Kim JS, Hong YT. Perceptual and acoustic outcomes of early-stage glottic cancer after laser surgery or radiotherapy: a meta-analysis. Clin Exp Otorhinolaryngol 2019;12(3):241-8.
34. Al-Mamgani A, Kwa SL, Tans L, Moring M, Fransen D, Mehilal R, et al. Single vocal cord irradiation: image guided intensity modulated hypofractionated radiation therapy for T1a glottic cancer: early clinical results. Int J Radiat Oncol Biol Phys 2015;93(2):337-43.
35. Yeo SG. Volumetric modulated arc radiotherapy of the whole larynx, followed by a single affected vocal cord, for T1a glottic cancer: dosimetric analysis of a case. Mol Clin Oncol 2016;4(3):429-32.
36. Osman SO, Astreinidou E, de Boer HC, Keskin-Cambay F, Breedveld S, Voet P, et al. IMRT for image-guided single vocal cord irradiation. Int J Radiat Oncol Biol Phys 2012;82(2):989-97.
|
|
|||||||||||||||||||||||||||||||||||||||